protoms.py¶
This program is used to setup a ProtoMS simulation. It was made with usability at highest priority. The only input that should be necessary is a couple of prepared PDB files containing the molecules one would like to simulate.
The program will create force field for small molecules, setup the protein and solvate the prepared system. At the moment it can setup the following types of simulations:
- Equilibration
- Sampling
- Dual-topology free energy
- Single-topology free energy
- Grand Canonical Monte Carlo (GCMC)
- Just Add Waters, stage 1 and 2 (JAWS-1, JAWS-2)
The program will create files and inputs based on experience that should work in most situations. However there might be situations where the created settings are not appropriate. One can then use individual tools to make a more custom setup, see this. One might also have to edit the files manually.
Syntax:
protoms.py [-s none|equilibration|sampling|dualtopology|singletopology|gcmc|jaws1|jaws2] [-f folder1 folder2] [-p protein.pdb] [-sc scoop.pdb] [-l lig1.pdb lig2.pdb ...] [-t template1 template2 ...] [-w water.pdb] [-c cmdfile] [-r nrepeats | prefix] [--outfolder folder] [--atomnames namefile] [--watmodel tip4p|tip3p] [--waterbox watbox] [--charge charge1 charge2] [--singlemap mapfile] [--center cent] [--innercut icut] [--outercut ocut] [--flexin sidechain|flexible|rigid] [--flexout sidechain|flexible|rigid] [--scooplimit N] [--capradius radius] [--lambdas nlambdas | lambda1 lambda2 ...] [--adams B1 B2 ...] [--jawsbias bias] [--gcmcwater wat.pdb | N] [--gcmcbox box.pdb | X Y Z A B C] [--nequil N] [--nprod N] [--dumpfreq N] [--absolute] [--dovacuum] [--testrun] [--cleanup]
-s none|equilibration|sampling|dualtopology|singletopology|gcmc|jaws1|jaws2= the type of simulation to perform- optional, default =
none
-f folder1 folder2= name of folders to search for input files- optional, no default
-p protein.pdb= the name of the protein PDB file- optional, no default
-o scoop.pdb= the name of a protein scoop PDB file- optional, no default
-l lig1.pdb lig2.pdb ...= the name(s) of PDB file(s) containing ligand(s)- optional, no default
-t template1 template2 ...= the name(s) of ProtoMS template file(s) that needs to be loaded- optional, no default
-w water.pdb= the name of a PDB file with bulk water for the protein- optional, no default
-c cmdfile= the prefix for the created ProtoMS command file- optional, default =
run
-r nrepeats | prefix= setup independent repeats of the simulation- optional, default = 1
nrepeat= repeats a created from 1 tonrepeatprefix= a single repeat is created, but prefix is appended to folders and files
--outfolder folder= the ProtoMS output folder- optional, default =
""(empty string)
--atomnames namefile= the name of file containing conversion instructions- optional, no default
if not given, takes the one in
$PROTOMSHOME/data
--watermodel tip4p|tip3p= the water model to use- optional, default =
tip4p
--waterbox watbox= the name a of a PDB file with a pre-equilibrated water box- optional, no default
if not given, takes one in
$PROTOMSHOME/data
--charge charge1 charge2… = the charges of the ligands- optional, default = 0
--singlemap mapfile= the correspondence map for single-topology setup- optional, no default
--center cent= the centre of the scoop- optional, default = 0.0,0.0,0.0
--innercut icut== the inner region cut-off in Angstroms- optional, default = 16.9 A
--outercut ocut== the outer region cut-off in Angstroms- optional, default = 20.0 A
--flexin sidechain|flexible|rigid= determine the flexibility of the inner region- optional, default =
flexiblesidechain= only the sidechains will be sampled in the simulationflexible= both sidechain and backbone will be sampled in the simulationrigid= no residues will be sampled
--flexout sidechain|flexible|rigid= determine the flexibility of the outer region- optional, default =
sidechainsidechain= only the sidechains will be sampled in the simulationflexible= both sidechain and backbone will be sampled in the simulationrigid= no residues will be sampled
--scooplimit N= the minimum removed number of residues in a scoop- optional, default = 10
--capradius radius= the radius of the droplet solvating the protein- optional, default = 30
--lambdas nlambdas | lambda1 lambada2… = specification of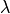 ; space for free energy calculations
; space for free energy calculations- optional, default = 16
if a single value is given, this number of
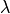 -values is created uniformly from 0 to 1
if a list of values are given, this is the
-values is created uniformly from 0 to 1
if a list of values are given, this is the 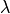 -values to use
-values to use
--adams B1 B2… = the Adams parameter for GCMC- optional, default = 0
--jawsbias bias= the bias to apply in JAWS-2 simulations- optional, default = 0
--gcmcwater wat.pdb | N= the name of a PDB file with reservoir waters for GCMC and JAWS-1 or an integer- optional, no default if an integer is given this corresponds to the number of water to add to the GCMC/JAWS-1 box
--gcmcbox box.pdb | X Y X A B C= the name of a PDB file with GCMC or JAWS-1 simulation box dimension or the box dimensions- optional, no default if six numbers are given this corresponds to the origin (first three) and the length (last three) of the box
--nequil N= the number of equilibration moves- optional, default = 5E6
--nprod N= the number of production moves- optional, default = 40E6
--dumpfreq N= the frequency with which output is written to disc- optional, default = 1E5
--absolute= turns on the setup of absolute free energies- optional, default = off
--dovacuum= turns on the setup of vacuum simulation- optional, default = off
--testrun= turns on the setup of a short simulations appropiate for tests- optional, default = off
--cleanup= cleans up extraenous files and put them in a tar-ball- optional, default = off
Examples:
protoms.py
protoms.py.py -s sampling -l lig1.pdb --dovacuum --testrun
protoms.py -s dualtopology -l lig1.pdb lig2.pdb -p protein.pdb
protoms.py -s dualtopology -l lig1.pdb --absolute
protoms.py -s gcmc -p protein.pdb --adams -4 -2 0 2 4 6
Notes:
The program will try to locate previously created files for the protein and ligand in the current working directory or any folder specified with the -f flag. For ligands the program will replace .pdb with the appropriate ending, such as .prepi for Amber prepi files and .tem for ProtoMS template files.
Starting with just the PDB-files of the ligand(s) and the protein, the program will create the following files in the same folder as those PDB-files
lig.prepi= the z-matrix and atom types of the ligand in Amber formatlig.frcmod= additional parameters not in GAFFlig.zmat= the z-matrix of the ligand used to sample it in the MC simulationlig.tem= the complete template (force field) file for the ligand in ProtoMS formatli1-li2.tem= the combined template file of all ligands- the filename is a combination of the residue name of all ligands
lig_box.pdb= the box of water solvating the ligandprotein_scoop.pdb= the truncated protein structureprotein_pms.pdb= the original protein structure with ProtoMS naming convention- if the scoop removes to few residues, this file be created instead
water.pdb= the cap of water solvating the protein system
In addition, for dual-topology simulations the following files are created: :
lig1_dummy.pdb= the dummy particle that the ligand will be perturbed to- only created if the –absolute flag is set
In addition, for single-topology simulations the following files are created:
li1-li2_ele.tem= the ProtoMS template file for electrostatic single-topology perturbationli1-li2_vdw.tem= the ProtoMS template file for van der Waals single-topology perturbationli1-li2_comb.tem= the ProtoMS template file for combined/single-step single-topology perturbationsettings.singlemap= the created correspondance map for single topology- only named like this if the –singlemap argument is not set
In addition, for GCMC / JAWS-1 simulations the following files are created:
gcmc_box.pdb/jaws1_box.pdb= the GCMC / JAWS-1 simulation boxgcmc_wat.pdb= the GCMC / JAWS-1 reservoire waterswater_clr.pdb= the cap of water solvating the protein system, cleared from the GCMC / JAWS-1 simulation box
In addition, for JAWS-2 simulations the following files are created:
jaws2_watN.pdb= the JAWS-2 water- each of the water given with the
--gcmc_waterflag will be written to an individual file
jaws2_notN.pdb= the rest of the JAWS-2 waterwater_clr.pdb= the cap of water solvating the protein system, cleared from the GCMC / JAWS-1 simulation box
It will create at most three ProtoMS command files, one for the protein simulation, one for the ligand simulation and one for the gas-phase simulation. These can be used to run ProtoMS, e.g.
$PROTOMS/protoms3 run_free.cmd
Prerequisites:
The program assumes that both the ligand and the protein is prepared before. This includes for instance protonation. At the moment only Amber naming convention is supported.
The progam requires AmberTools to make force field for small molecules.
